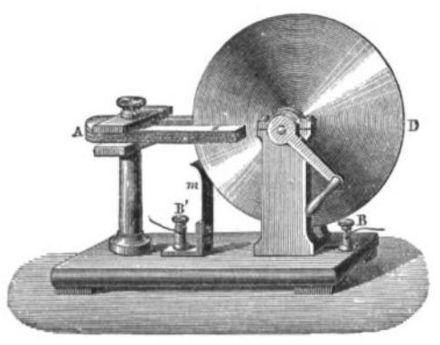
In 1752, Benjamin Franklin conducted his famous kite and key experiment that proved lightning and electric sparks were the same thing. He is widely considered to be the first person in recent times to discover electricity. However, it wasn’t until 1831, when Michael Faraday invented the dynamo generator (Faraday Disk) that electricity could be used commercially. Faraday’s invention allowed humans to create a continuous source of electricity.
Later on, prominent figures such as Nikola Tesla and Thomas Edison developed even more useful applications for electricity. Edison developed the first filament light bulb, as well as the DC current supply system. Tesla is best known for the invention of the modern day AC poly-phase electrical transmission and distribution system.
Charges and the Electrostatic Force
The simplest definition of electricity is the flow of charge.
But what is charge? Charge is a physical property of matter, caused by a difference between the number of protons and electrons in a particle. A charged particle will experience a force when placed in an electromagnetic field. Charges can be positive or negative, depending on the balance of protons (+e) and electrons (-e).
Charge is usually denoted with a “q”, and is measured in Coulombs (C). The Coulomb is named after French physicist Charles-Augustin de Coulomb, who discovered what is now known as Coulomb’s Law which describes the electrostatic force of attraction and repulsion. One electron has 1.602 × 10-19 C of charge which means that one Coulomb of charge contains 6.24 x 1018 electrons.
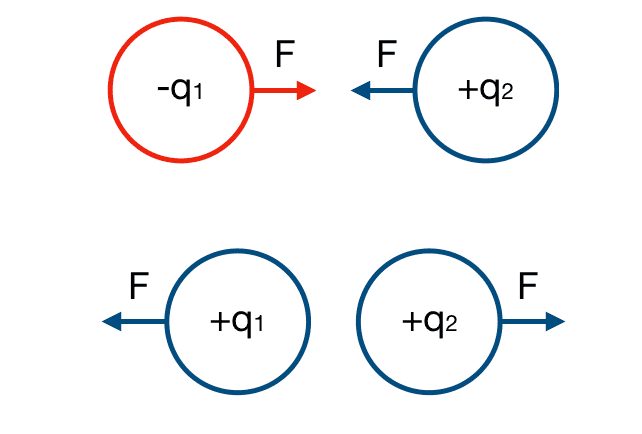
Opposite charges attract, while like charges repel. This is known as the electrostatic force. The electrostatic force is the attractive or repulsive forces between two charged objects. The force between these particles can be calculated with:
Where q1 and q2 are the respective charges, F is the force between the charges, r is the distance between the charges in meters, and k is Coulomb’s constant (9.0 x 109 N • m2 / C2 ).
Electrons
We know that electricity is the flow of charge and that electrons contain a negative charge of 1.602 × 10-19 C. But what is an electron?
Electrons are negatively charged subatomic particles that are found orbiting the nuclei of atoms. A nucleus is typically made up of neutrons and protons. Neutrons maintain a neutral charge while protons maintain a positive charge.
In the diagram above, the black particles are electrons, the blue particles are neutrons, and the red particles are protons. This diagram is not to scale. Electrons are tiny (1/1840 the mass) compared to protons and neutrons.
In an uncharged atom, the number of electrons equals the number of protons.
So what do electrons have to do with electricity?
The diagram above shows the Bohr atomic model of Aluminium. This diagram illustrates the arrangement of electrons in “shells” surrounding the nucleus. Electrons in the outermost shell are known as valence electrons, which can leave the atom with sufficient energy in order to become free. These free electrons are able to move through a conductor and create electricity.
Electric Fields
Electric fields are responsible for the force that generates the flow of charges (electricity). An electric field is created by a charged particle. An electric field can exert a force on other charged particles. Electric fields are defined as the amount of electrostatic force per unit charge.
A negative charge has inward pointing electric field lines, while a positive charge has outward pointing electric field lines. The electric field lines indicate the direction of force the charges would exert on a positive charge. A negative charge would attract a positive charge, while a positive charge would repel a positive charge.
Electromagnetic Fields
An electromagnetic field (EMF) is produced by moving charges. These fields are a combination of electric and magnetic fields. While electric fields are created by stationary charges, magnetic fields are created by moving charges. Electromagnetic fields exist all around us. The electromagnetic spectrum includes everything from radio waves to gamma rays, and even visible light!
Electric Potential Energy
Electric potential energy is defined as the energy required to assemble a system of charges. In other words, a charge’s electric potential describes how much energy it has stored. This is similar to gravitational potential energy and kinetic energy. A stationary object at a particular height has more gravitational potential energy than kinetic energy. But if the object is allowed to fall, the gravitational field will convert the gravitational potential energy into kinetic energy. Similarly, once a charged particle enters an electrostatic field, its electric potential energy is converted into kinetic energy.


Superb piece👌
Excellent article!